Abstract
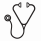
Background. Chronic active BCR or TCR signaling or its oncogenic mimics result in nuclear accumulation of NF-kB. Oncogenic mimicry of BCR- and TCR signaling can be induced by viral oncoproteins (e.g. LMP2A (EBV), K1 (KSHV) and Tax (HTLV1), activating mutations in the CD79A, CD79B and CD3Z signaling chains or genetic lesions driving tyrosine kinase (e.g. BCR-ABL1), ERK (RAS, BRAF, PLCG1) or PI3K (PIK3CA, PIK3R1) signaling. While BCR and TCR signaling induces positive selection, survival and proliferation in normal lymphocytes, the majority of B cell and T cell malignancies are driven by oncogenic activation of this pathway.
Concept & central hypothesis. Oncogenic drivers in B- and T-lymphoid malignancies function as mimics of B- and T-cell receptor (BCR or TCR) signaling. Oncogenic activation of BCR- or TCR-signaling represents the functional equivalent of positive selection during normal lymphocyte development. Addiction to survival and proliferation signals (or the equivalent of positive selection) is a common feature in many types of cancer. However, B- and T-lymphoid malignancies are unique in that they are also subject to an active negative selection process. B- and T-cells expressing autoreactive antibodies and TCRs, respectively, can cause systemic autoimmunity. As a safeguard against autoimmune diseases, lymphocyte development evolved autoimmunity checkpoints (AIC) to eliminate autoreactive clones . Owing to negative selection of autoreactive B- and T-cells through AIC activation, lymphoid cells fundamentally differ in their signaling requirements from other cell types. Four recent studies from our group showed that despite malignant transformation, B cell- and T cell-lineage leukemia and lymphoma cells are fully sensitive to negative selection and AIC-activation resulting (Chen et al., Nature 2015; Shojaee et al., Cancer Cell 2015; Shojaee et al., Nature Med 2016; Chan et al., Nature 2017).
Results: AIC-activation in various lymphoid malignancies is achievable by pharmacological hyperactivation of BCR- and TCR-signaling above a maximum threshold, e.g. pharmacological inhibition of PTEN, SHIP1 or DUSP6 phosphatases (Figure). Studying genetic models of B cell malignancies, we observed that Cre-mediated deletion of Pten, Ship1 and constitutive deletion of Dusp6 caused hyperactivation of SYK, PI3K and ERK-pathways, leading to AIC-activation. Unlike other types of cancer, B- and T-cell malignancies are uniquely susceptible to clonal deletion induced by hyperactive signaling from an autoreactive BCR or TCR. Hence, targeted AIC-activation can be leveraged for eradication of drug-resistant leukemia and lymphoma clones. Studying engineered B cells for inducible activation of autoreactive BCR-signaling, we discovered that targeted hyperactivation of SYK, PI3K and ERK in B cell malignancies represents the functional equivalent of an autoimmunity checkpoint (AIC) for elimination of autoreactive clones. Likewise, transformed B cell tumors are uniquely vulnerable to AIC activation, suggesting that targeted activation of this checkpoint represents a novel strategy to induce cell death in otherwise drug-resistant B cell malignancies.
Conclusion: Normal B- and T-cells are positively selected for BCR or TCR signaling of intermediate strength (moderate activation of SYK, PI3K and ERK). In the absence of a functional BCR/TCR, SYK, PI3K and ERK activity fall below a minimum threshold, resulting in death by neglect. Hyperactivation above maximum thresholds (e.g. autoreactive BCR/TCR) triggers negative selection and cell death via AIC-activation. Targeted therapy of cancer typically focuses onagents that suppress oncogenic signaling below a minimum threshold . Our results support a novel strategy to overcome drug-resistance in B- and T-cell malignancies based on targeted activation of autoimmunity checkpoints (AIC) for removal of autoreactive cells.
Muschen: Pfizer: Research Funding; AbbVie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal